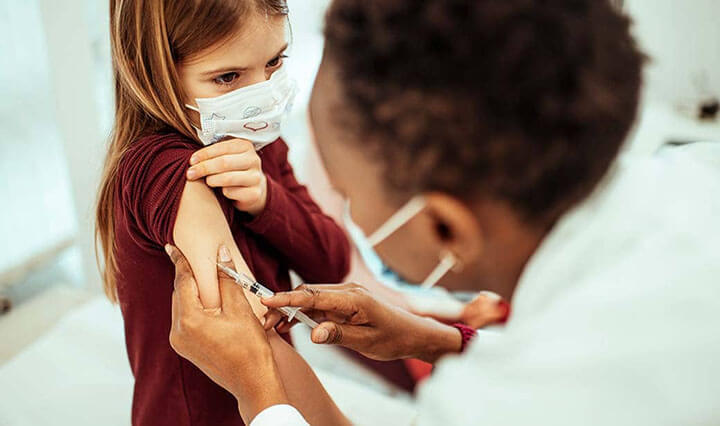
It’s the first shot kids might actually like.
A panel of researchers commissioned by the Food and Drug Administration voted Tuesday to approve the Covid-19 vaccine developed by Pfizer / BioNTech for emergency use in children ages 5 to 11.
The ruling clears the way for millions of youngsters to protect themselves and others from the sometimes deadly virus.
First jabs could be in arms as early as next week.
The advisory panel voted unanimously (with one abstention), concluding that the vaccine’s benefits in preventing COVID-19 outweigh any potential risks.
Pfizer shots have been proven to be nearly 91% effective at preventing symptomatic infection, according to published reports — even though the elementary school set is administered just one third of the dose given to those 12 and up.
Leaders of the FDA still must officially sign off before parents can rush to the pediatrician’s office — but that green light is expected in the next 24 hours.
The process would move to the Centers for Disease Control and Prevention next Tuesday.
“If all goes well, and we get the regulatory approval, and the recommendations from the CDC, it’s entirely possible, if not, very likely, that vaccines will be available for children from 5 to 11 within the first week or two of November,” Dr. Anthony Fauci, noted in an interview last Sunday on ABC’s This Week.
Don’t miss out on a single story!
Follow us on Twitter * Follow us on Facebook * Submit a news tip
Check out our complete story archive
- R.I.P.:Unleashed By Petco set to close - February 18, 2026
- Hotsheet 2/19/26: Will SMMUSD eliminate teachers? - February 17, 2026
- Hotsheet 2/5/26:SMC in ‘financial crisis’; Cat cafe opens - February 5, 2026



